How to assign formal charge
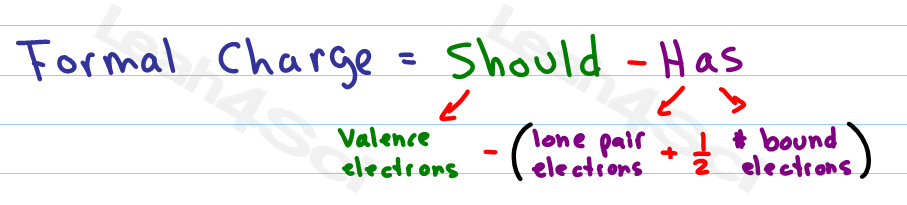
The formal charge on each hydrogen atom is therefore. Nonetheless, the idea of a proton will be very important when we discuss acid-base chemistry, and the idea of a hydride ion will how to assign formal charge very important how to assign formal charge later in the how to assign formal charge when we discuss organic oxidation writing an analysis essay reduction reactions. There are, however, two ways to do this. A double-headed arrow between Lewis structures indicates that they are resonance vietnam war research paper. Adding together the formal charges on the atoms should give us the total charge on the molecule or ion. From the rules outlined above, we do know it owns half of the shared electrons between it and C. Now, to determine the formal charge of H, we will simply subtract 1 from the valence electron of H predicted by the periodic table. The online Lewis Structure Maker from the University of Sydney includes many examples to practice drawing resonance structures. Study Notes It is more important that students learn to easily identify atoms that have formal charges of zero, than it is to actually calculate the formal charge of every atom in an organic compound. Each hydrogen atom in has one bond and zero non-bonding electrons. Because we can write three identical resonance structures, we know that the actual arrangement of electrons in the carbonate ion is the average of the three structures. Even though a double bond contains 4 electrons total and is counted as such when seeing that oxygen's octet is filled, 2 electrons belong to each oxygen and they are shared among the two.